Abstract
Introduction
Decision-making in the treatment (Tx) of chronic myeloid leukemia (CML) is complex and dependent on many factors, including disease phase and drug tolerability. The tyrosine kinase inhibitor (TKI) dasatinib is an effective long-term Tx option for most patients (pts) with newly diagnosed CML, based on the deep and durable responses reported in the DASISION trial (Cortes JE, et al. J Clin Oncol 2016); however, dasatinib was associated with the development of pleural effusion, an adverse event caused by the build-up of excess fluid in the pleural space outside of the lungs (Jany and Welte Dtsch Arztebl Int 2019). Data regarding the optimal strategy for managing pleural effusion among pts treated with dasatinib are limited. Dose reductions, interruptions or switching to another TKI are commonly used strategies but in the SIMPLICITY trial, pts who remained on first-line Tx had better clinical outcomes than pts who switched Tx (Gambacorti-Passerini C, et al. Eur J Haematol 2020). Here we present results of a study examining Tx patterns, including duration of dasatinib use after pleural effusion, and healthcare resource utilization (HCRU) and costs among pts with CML treated with dasatinib who experienced a subsequent pleural effusion.
Methods
Data from the IBM MarketScan ® Commercial and Medicare Supplemental Databases were used to identify pts diagnosed with CML (ICD-9-CM code 205.1x, 205.8x; ICD-10-CM code C92.1x, C92.Zx) during the study period (Jan 1, 2014 - Sep 30, 2019). Eligible pts were ≥ 18 y, had ≥ 1 pharmacy claim for dasatinib, and experienced a pleural effusion after dasatinib Tx (ICD-9-CM: 511.1, 511.89; ICD-10-CM: J90) during the identification (ID) period (Jan 1, 2015 - Sep 30, 2018). All pts had ≥ 1 filled prescription for dasatinib before the index date (defined as date of first pleural effusion during ID period), with dasatinib available on the index date, and no code for pleural effusion recorded during the baseline period (1-y pre-index); pts were also required to be continuously enrolled (baseline to follow-up at 1-y post-index). Demographic and clinical characteristics were described, and endpoints evaluated included Tx patterns (dose modification, switching to another TKI, duration of dasatinib Tx), HCRU, and cost.
Results
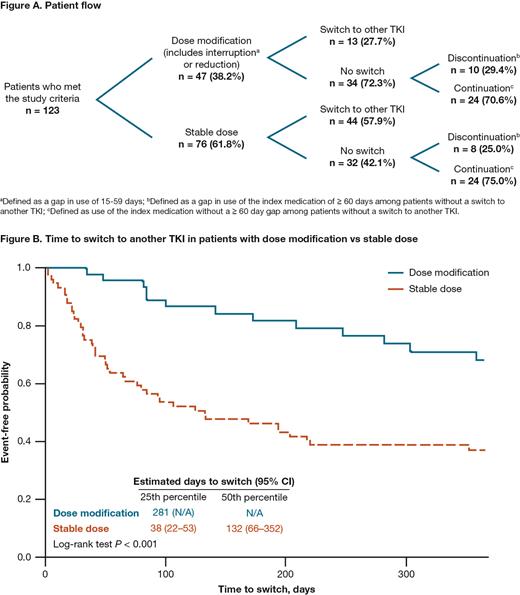
In total, 123 pts met the study criteria. The mean (standard deviation [SD]) age was 62.2 (10.9) y, 23.6% were female, and the mean (SD) Charlson Comorbidity Index was 3.8 (2.1). Overall, 38.2% of pts had a dose modification and 61.8% a stable dose after pleural effusion. At the 1-y follow-up, most pts (72.3%) with a dose modification did not switch Tx, and, of those, 70.6% continued Tx whereas the majority (57.9%) of pts with a stable dose switched to another TKI (Figure A). The mean (SD) number of days from first pleural effusion to end of dasatinib Tx (duration of dasatinib Tx) was significantly greater in pts with a dose modification compared with those with a stable dose (262.0 [124.0] vs 149.1 [155.2]; P < 0.001). In pts with a dose modification, the mean (SD) number of days from pleural effusion to dose modification was 73.7 (77.1) days, and from dose modification to end of Tx was 188.3 (128.7) days. Pts with a dose modification took a significantly longer time to switch from dasatinib to another TKI compared with pts with a stable dose (mean [SD]: 164.7 [105.8] vs 74.8 [76.0] days; P < 0.001; Figure B). Overall, 48.0% of pts had an inpatient hospitalization, with a mean (SD) total stay of 11.3 (14.3) days, and 37.4% had a visit to an emergency department. The mean (SD) number of physician office visits was 24.3 (16.7). Total mean (SD) costs were $196,797 (143,848). There were no statistically significant differences in HCRU and costs between pts with dose modification and stable dose.
Conclusions
These findings demonstrate that dasatinib discontinuation may not be necessary after development of pleural effusion. Pts who had a dose modification of dasatinib after development of pleural effusion were able to continue on dasatinib for a longer duration and had a lower rate of switching to another TKI but with similar HCRU and costs compared with pts who maintained a stable dose. Although not all pts required a dose modification to continue dasatinib Tx after pleural effusion, these findings suggest that in some pts, dose modification of dasatinib may allow for continued Tx with the potential to sustain outcomes.
Study support
Funded by Bristol Myers Squibb
Brokars: Bristol Myers Squibb: Current Employment. Kee: Bristol Myers Squibb: Current Employment. McBride: Bristol Myers Squibb: Current Employment. Reddy: Amgen: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Partnership for Health Analytic Research (PHAR), LLC: Current Employment; Greenwich Biosciences: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; GRAIL: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Prothena: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Jazz: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Kite: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Genentech: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Verde Technologies: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Exact Sciences Corporation: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Eisai: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Dompe: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Celgene: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Sage: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Otsuka: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Novartis: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Mirum Pharmaceuticals: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Boston Scientific Corporation: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Bristol Myers Squibb: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Sanofi US Services: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Takeda Pharmaceuticals: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Akcea: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; BioMarin Pharmaceutical: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months. Chang: AstraZeneca: Other: I am an employee of PHAR, LLC, which was paid by AstraZeneca to conduct research; Bristol Myers Squibb: Other: I am an employee of PHAR, LLC, which was paid by BMS to conduct research; Boston Scientific Corporation: Other: I am an employee of PHAR, LLC, which was paid by Boston Scientific Corporation to conduct research; Celgene: Other: I am an employee of PHAR, LLC, which was paid by Celgene to conduct research; Eisai: Other: I am an employee of PHAR, LLC, which was paid by Eisai to conduct research; Ethicon: Other: I am an employee of PHAR, LLC, which was paid by Ethicon to conduct research; GRAIL: Other: I am an employee of PHAR, LLC, which was paid by GRAIL to conduct research; Helsinn: Other: I am an employee of PHAR, LLC, which was paid by Helsinn to conduct research; Illumina: Other: I am an employee of PHAR, LLC, which was paid by Illumina to conduct research; Ionis: Other: I am an employee of PHAR, LLC, which was paid by Ionis to conduct research; Jazz: Other: I am an employee of PHAR, LLC, which was paid by Jazz to conduct research; Kite: Other: I am an employee of PHAR, LLC, which was paid by Kite to conduct research; Novartis: Other: I am an employee of PHAR, LLC, which was paid by Novartis to conduct research; Otsuka: Other: I am an employee of PHAR, LLC, which was paid by Otsuka to conduct research; Pathnostics: Other: I am an employee of PHAR, LLC, which was paid by Pathnostics to conduct research; Prothena: Other: I am an employee of PHAR, LLC, which was paid by Prothena to conduct research; Sage: Other: I am an employee of PHAR, LLC, which was paid by Sage to conduct research; Verde Technologies: Other: I am an employee of PHAR, LLC, which was paid by Verde Technologies to conduct research; Genentech, Inc.: Other: I am an employee of PHAR, LLC, which was paid by Genentech to conduct research; Greenwich Biosciences, Inc.: Other: I am an employee of PHAR, LLC, which was paid by Greenwich Biosciences to conduct research; Mirum Pharmaceuticals, Inc.: Other: I am an employee of PHAR, LLC, which was paid by Mirum Pharmaceuticals, Inc. to conduct research; Dompe US, Inc.: Other: I am an employee of PHAR, LLC, which was paid by Dompe US, Inc. to conduct research; Sanofi US Services, Inc.: Other: I am an employee of PHAR, LLC, which was paid by Sanofi US Services Inc. to conduct research; Sunovion Pharmaceuticals, Inc.: Other: I am an employee of PHAR, LLC which was paid by Sunovion Pharmaceuticals, Inc. to conduct research. ; BioMarin Pharmaceuticals Inc.: Other: I am an employee of PHAR, LLC which was paid by BioMarin Pharmaceuticals, Inc. to conduct research. ; Takeda Pharmaceuticals U.S.A., Inc.: Other: I am an employee of PHAR, LLC which was paid by Takeda Pharmaceuticals U.S.A., Inc., to conduct research. ; Exact Sciences Corporation: Other: I am an employee of PHAR, LLC which was paid by Exact Sciences Corporation to conduct research. ; Amgen: Other: I am an employee of PHAR, LLC, which was paid by Amgen to conduct research; Akcea: Other: I am an employee of PHAR, LLC, which was paid by Akcea to conduct research; AbbVie: Other: I am an employee of PHAR, LLC, which was paid by AbbVie to conduct research; Partnership for Health Analytic Research (PHAR), LLC: Current Employment, Other. Tarbox: Partnership for Health Analytic Research (PHAR), LLC: Current Employment, Other: I am an employee of PHAR, LLC, which was paid by Celgene/BMS to conduct this research.; AbbVie: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Akcea: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Amgen: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; BioMarin Pharmaceuticals: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Bristol Myers Squibb: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Boston Scientific Corporation: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Celgene: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Dompe: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Eisai: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Exact Sciences Corporation: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Genentech: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; GRAIL: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Greenwich Biosciences: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Jazz: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Kite: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Mirum Pharmaceuticals: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Novartis: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Otsuka: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Prothena: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Sage: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Sanofi US Services: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Takeda Pharmaceuticals USA: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months; Verde Technologies: Other: I am an employee of PHAR, LLC, which was paid by this company to conduct research in the last 24 months. LeBlanc: Jazz Pharmaceuticals: Research Funding; Daiichi-Sankyo: Consultancy, Honoraria, Other: Advisory board; Astellas: Consultancy, Honoraria, Other: Advisory board; AstraZeneca: Consultancy, Honoraria, Other: Advisory board, Research Funding; Amgen: Consultancy, Other: travel; American Cancer Society: Research Funding; CareVive: Consultancy, Other, Research Funding; Flatiron: Consultancy, Other: Advisory board; Pfizer: Consultancy, Other: Advisory Board; Helsinn: Consultancy, Research Funding; BMS/Celgene: Consultancy, Honoraria, Other: Travel fees, Research Funding, Speakers Bureau; Seattle Genetics: Consultancy, Other: Advisory board, Research Funding; Otsuka: Consultancy, Honoraria, Other; Duke University: Research Funding; UpToDate: Patents & Royalties; NINR/NIH: Research Funding; Agios: Consultancy, Honoraria, Other: Advisory board; Travel fees, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Advisory board; Travel fees, Speakers Bureau; Heron: Consultancy, Honoraria, Other: advisory board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal